Improving the physical characteristics of metal components often requires fine-tuned treatments that bring them to the brink of destruction. It’s a quirk of metallurgy heat treaters contend with constantly.
Several years ago, a longtime customer asked us to investigate the thermal processing of constant tension bands it supplied to major American automotive manufacturers. Heat treating these parts was complex. Even the slightest out-of-spec conditions could result in failed quality tests.
In this case, we needed to figure out how to reduce the risk that the constant tension bands would fail due to stress corrosion cracking.
After monitoring countless variables during our rigorous investigation, we broke a few cardinal rules en route to refining the heat treating process and improving the quality of the bands.
Inherent risk in a severe service environment
ASM International defines stress corrosion cracking as “a failure process that occurs because of the simultaneous presence of tensile stress, an environment and a susceptible material. The failures often take the form of fine cracks that penetrate deeply into the metal, with little or no evidence of corrosion on the nearby surface or distortion of the surrounding structure.”
The constant tension bands our customer manufactured were designed to ensure that rubber hoses in cars and trucks keep a snug fit. They were made of 6150 spring steel, the default alloy used for this application.
But under the hood or beneath the chassis is a rough service environment featuring corrosives such as road salt and water. The criteria for elevated stress corrosion cracking risk are met in this environment:
- 6150 steel is susceptible to stress corrosion cracking.
- A corrosion source is present.
- The part is under stress.
Austempering 6150 constant tension bands
Part specifications required that the constant tension bands be austempered and then quenched in a molten salt bath targeting a hardness around 50 HRC.
Austempering produces bainite, a structural characteristic of steel known to be less susceptible to stress corrosion cracking. To ensure the part retains that structure while reaching the target hardness, we had to set our salt baths to a temperature just ten degrees above the 6150 steel’s martensitic start point. The martensitic start point is temperature at which the structure of the cooling steel would begin to transition from bainite to martensite. We had to avoid this transition because martensite is associated with greater stress corrosion cracking risk.
In other words, successful heat treatment required process parameters that came critically close to circumstances that would result in failed quality tests and possible part failure.
It’s the metallurgical equivalent of threading a needle, so we conducted frequent stress tests on the parts. If constant tension bands don’t perform as intended, hoses may slip loose from their connections resulting in serious engine problems.
In this case, testing revealed more batches required reprocessing than the customer would tolerate. No safety-critical tests came back as failures and no issues had been flagged by automakers or drivers, but a constant fear still loomed over our customer.
A faulty sensor, intentional rule-breaking and a problem solved
OEM specs required that our customer develop an experiment to find out what was happening. It was up to us to carry out the tests. Getting it right meant monitoring and noting thousands of process variables.
First, after the parts were heat treated as specified, they were placed on a plastic mandrel that applied a load near their yield stress level.
Next, the parts were submerged in a concentrated hydrochloric acid solution. If parts didn’t snap before eight minutes elapsed, it was a success.
The testing progressed normally, until one day, nothing went wrong.
It was a good thing we kept such a close eye on process parameters. Poring over all the data we collected during the heat treatment and stress corrosion cracking test, we discovered an anomaly: The true amount of carbon present in the furnace during austempering was less than the indicated amount. A faulty sensor was to blame for the carbon deficit despite our team calibrating the furnace to the correct set point.
This is a critical detail. Metallurgical doctrine states that to achieve our target characteristics for the 6150 spring steel we used, the carbon set point in the furnace must match the carbon content of the steel. Equilibrium between the part and the atmosphere ensures the part’s carbon content doesn’t change during treatment.
The low actual carbon set point created a mismatch that resulted in a slight decarburization of the steel—some carbon had been drawn out of the part.
Our metallurgists were puzzled. Decarburization in this application is a no-no because it alters the martensitic start point, increasing the chances that martensite will form. Recall that formation of martensite for this application was understood to be bad.
Actually, it looked really good. We began experimenting with “incorrect” treatment conditions, purposely lowering the carbon set point. The part did not fail its stress corrosion cracking test.
We did more process tweaking, too, including austempering above the specified 1,550 degrees Fahrenheit. That resulted in improved dissolution of primary carbides in the part.
To confirm we’d discovered a process that would result in far fewer stress corrosion cracking test failures, we beefed up the parameters of our internal tests. Instead of monitoring for failures after eight minutes submerged in the hydrochloric acid solution, we bumped it up to 12 minutes.
Still no failures.
Why we think it worked
We suspect the improved mechanical characteristics we observed were a result of this sequence of events:
- When the lower carbon set point in the furnace caused slight decarburization of the part, it set the stage for the formation of martensite during quenching.
- Martensite expands when it forms and takes up more space than bainite.
- Formation of martensite likely put compressive stress on the part surface.
- This added compressive stress would lower the operational stress on the part surface, mitigating one of the three conditions known to cause stress corrosion cracking.
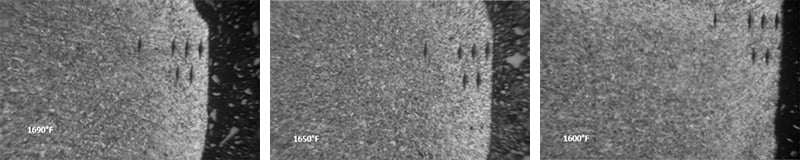
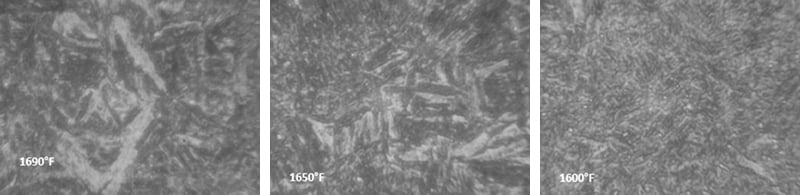
Below are photographs taken under microscope showing the structure of constant tension bands treated at different temperatures.
First, a 50x magnified surface view of parts austempered at 1690, 1650 and 1600 degrees Fahrenheit:
Next, a 400x magnified view near the surface of the parts austempered at the different temperatures:
Finally, a 1000x magnified view of the cores of the austempered parts:
After settling on a new heat treatment recipe, we brought it to the customer. They approved our changes based on the compelling data we collected. Their business improved in two ways.
First, with far fewer stress test failures, far fewer batches required rework, ultimately lowering manufacturing costs. Second, the customer no longer lived in a state of constant fear.
Paulo solves mysteries daily
Is your current thermal processor unable to figure out why parts keep failing? Are you developing a totally new part or considering a part redesign?
Whether you need to get to the bottom of your most stubborn mysteries or push the envelope to see what’s possible, our metallurgists love playing detective. Getting answers starts by getting in touch with our team. Contact us now.